Laboratory´s responsibility of POCT - example from Finland
Publikováno dne: 29. března 2016
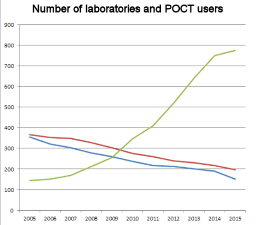
Point-of-care testing is spreading out everywhere in Europe. At the same time the number of clinical laboratories is decreasing due to consolidation and international private laboratory chains. Huge analyzers and automated sample processing make consolidation economically tempting, but everything cannot be centralized. Specimen collection and emergency analytics have to stay near patient. There is a great need for point-of-care testing and it is growing out of control. An increasing number of decisions concerning patient care and diagnosis are based on point-of-care test results. Therefore the results of rapid tests have to be as reliable and as accurate as traditional laboratory tests and the quality requirements have to be set on the same level. But how can we organize users training, orientation, instrument selection and validation, internal and external quality assessment? How we build the quality system and according to which standard? By means of legislation and recommendations point-of-care test users can be forced to use controls and participate to external quality assessment programs, but reporting and certifying the results from analytical phase of the test doesn´t tell the full story of the whole testing process. Most of the errors in point-of-care testing occur in preanalytical phase, especially in specimen collection and specimen handling. To be able to ensure the quality of point-of-care testing, we need trained laboratory professionals and multi-professional co-operation. |
Délka prezentace:
|
Klíčová slova: POCT, point-of-care-testing, quality assessment
Jak citovat toto dílo?
Juha Wahlstedt: Laboratory´s responsibility of POCT - example from Finland. PO>STUDIUM [online] 29. března 2016 , poslední aktualizace 29. března 2016 [cit.
]. Dostupný z WWW: https://postudium.lfp.cuni.cz/mod/data/view.php?d=13&mode=single&page=109&rid=403&filter=1. ISSN 1803-8999.
Přehledná publikace (Review)
Téma (obor) příspěvku:
Klinická biochemie
Analytika
Stav publikace: publikováno (send)